REVERSE ALZHEIMER’S SUMMIT 2022 was an online event held in July with some well-known speakers, some from alternative medicine circles. The event presented a plethora of alleged solutions to reverse one of the world's most dreadful diseases. As for remedies, you could...
HBOT listed as one of many promising therapies in search for Alzheimer’s cure
The big question everyone wants to know is, are we getting closer to curing Alzheimer's Disease? According to an article in the Daily Mail, the answer is yes. Hyperbaric Oxygen Therapy was one of several promising therapies outlined in the article, as scientists...
Alzheimer’s disease treatment: HBOT / Hyperbaric Oxygen Therapy
Beta-amyloid plaques (orange) clumping around brain cells (blue). Science Photo Library/Alamy Stock PhotoJune is Alzheimer’s and Brain awareness month - it is a time when we all work together to bring more awareness to the detrimental cognitive decline often...
Study indicates efficacy of hyperbaric oxygen therapy in reversing Alzheimer’s activators
A new study, published in the peer-reviewed medical journal Aging, marks the first time non-pharmaceutical clinical exploration has proved efficacious in reversing the main activators of Alzheimer’s disease. Using a specific protocol of hyperbaric oxygen therapy...
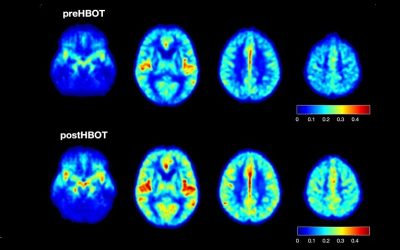
Hyperbaric oxygen therapy for Alzheimer’s dementia with positron emission tomography imaging: a case report.
A 58-year-old female was diagnosed with Alzheimer’s dementia (AD) which was rapidly progressive in the 8 months prior to initiation of hyperbaric oxygen therapy (HBOT). Fluorodeoxyglucose (FDG) positron emission tomography (PET) brain imaging demonstrated global and typical metabolic deficits in AD (posterior temporal-parietal watershed and cingulate areas). An 8-week course of HBOT reversed the patient’s symptomatic decline. Repeat PET imaging demonstrated a corresponding 6.5-38% regional and global increase in brain metabolism, including increased metabolism in the typical AD diagnostic areas of the brain. Continued HBOT in conjunction with standard pharmacotherapy maintained the patient’s symptomatic level of function over an ensuing 22 months. This is the first reported case of simultaneous HBOT-induced symptomatic and FDG PET documented improvement of brain metabolism in AD and suggests an effect on global pathology in AD.
The treatment of cognitive dysfunction in dementia: a multiple treatments meta-analysis.
No cure is currently available for dementia; however, various treatments and interventions have been reported to be effective. The factors influencing the efficacy of dementia treatment have not been comprehensively evaluated. This study evaluated the factors influencing treatment effects on cognitive dysfunction in dementia by comparing the results obtained from a meta-analysis based on meta-regression. The most effective intervention for dementia available is symptomatic treatment for vascular dementia. Antipsychotic treatment for dementia alleviates cognitive dysfunction less effectively than does symptomatic treatment. Alternative therapies are also effective at present. Further research on causes and very early diagnosis of Alzheimer disease is warranted.
Hyperbaric Oxygen Therapy for Alzheimer’s Disease.
Recent studies indicate that hyperbaric oxygen therapy (HBOT), a well-established therapy for decompression illness, could be a potential therapy for Alzheimer’s disease (AD). However, due to oxygen toxicity i.e., increased oxidative stress implicated in HBOT, the risk and benefit of HBOT for AD patients need to be further assessed clinically.
Hyperbaric oxygen therapy ameliorates pathophysiology of 3xTg-AD mouse model by attenuating neuroinflammation.
There is a real need for new interventions for Alzheimer’s disease (AD). Hyperbaric oxygen therapy (HBOT), the medical administration of 100% oxygen at conditions greater than 1 atmosphere absolute, has been used successfully to treat several neurological conditions, but its effects on AD pathology have never been thoroughly examined. Therefore, we exposed old triple-transgenic (3xTg) and non-transgenic mice to HBOT followed by behavioral, histological, and biochemical analyses. HBOT attenuated neuroinflammatory processes by reducing astrogliosis, microgliosis, and the secretion of proinflammatory cytokines (IL-1β and TNFα) and increasing expression of scavenger receptor A, arginase1, and antiinflammatory cytokines (IL-4 and IL-10). Moreover, HBOT reduced hypoxia, amyloid burden, and tau phosphorylation in 3xTg mice and ameliorated their behavioral deficits. Therefore, we suggest that HBOT has multifaceted effects that reduce AD pathologies, even in old mice. Given that HBOT is used in the clinic to treat various indications, including neurological conditions, these results suggest HBOT as a novel therapeutic intervention for AD.
Hyperbaric Oxygen Pretreatment Improves Cognition and Reduces Hippocampal Damage Via p38 Mitogen-Activated Protein Kinase in a Rat Model.
To investigate the effects of hyperbaric oxygen (HBO) pretreatment on cognitive decline and neuronal damage in an Alzheimer’s disease (AD) rat model. Rats were divided into three groups: normal saline (NS), AD, and HBO+AD. In the AD group, amyloid β peptide (Aβ)₁₋₄₀ was injected into the hippocampal CA1 region of the brain. NS rats received NS injection. In the HBO+AD group, rats received 5 days of daily HBO therapy following Aβ₁₋₄₀ injection. Learning and memory capabilities were examined using the Morris water maze task. Neuronal damage and astrocyte activation were evaluated by hematoxylin-eosin staining and immunohistochemistry, respectively. Dendritic spine density was determined by Golgi-Cox staining. Tumor necrosis factor-α, interleukin-1β, and interleukin-10 production was assessed by enzyme-linked immunosorbent assay. Neuron apoptosis was evaluated by terminal deoxynucleotidyl transferase dUTP nick end labeling. Protein expression was examined by western blotting. HBO pretreatment improves cognition and reduces hippocampal damage via p38 MAPK in AD rats.
Hyperbaric Oxygen and Ginkgo Biloba Extract Ameliorate Cognitive and Memory Impairment via Nuclear Factor Kappa-B Pathway in Rat Model of Alzheimer’s Disease.
Hyperbaric oxygen (HBO) and Ginkgo biloba extract (e.g., EGB 761) were shown to ameliorate cognitive and memory impairment in Alzheimer’s disease (AD). However, the exact mechanism remains elusive. The aim of the present study was to investigate the possible mechanisms of HBO and EGB 761 via the function of nuclear factor kappa-B (NF-κB) pathway. AD rats were induced by injecting β-amyloid 25-35 into the hippocampus. All animals were divided into six groups: Normal, sham, AD model, HBO (2 atmosphere absolute; 60 min/d), EGB 761 (20 mg·kg-1·d-1 ), and HBO/EGB 761 groups. Morris water maze tests were used to assess cognitive, and memory capacities of rats; TdT-mediated dUTP Nick-End Labeling staining and Western blotting were used to analyze apoptosis and NF-κB pathway-related proteins in hippocampus tissues. orris water maze tests revealed that EGB 761 and HBO significantly improved the cognitive and memory ability of AD rats. In addition, the protective effect of combinational therapy (HBO/EGB 761) was superior to either HBO or EGB 761 alone. In line, reduced apoptosis with NF-κB pathway activation was observed in hippocampus neurons treated by HBO and EGB 761. ur results suggested that HBO and EGB 761 improve cognitive and memory capacity in a rat model of AD. The protective effects are associated with the reduced apoptosis with NF-κB pathway activation in hippocampus neurons.